ABSTRACT
This paper deals with the principles and techniques that need to be applied to the food industry for an effective management of each critical control point (CCP) found in the process or in the equipment under examination. The application (to an aseptic filler CCP) is shown, of the principles presented in the author’s paper, titled ‘How to Monitor Food Equipment Critical Parts to Design Reliable Maintenance Tasks’, published in Issue 14.4. The use is extended of Hazard Analysis and Critical Control Points (HACCP) principles, integrating them with Reliability Centred Maintenance technique to produce a maintenance approach guided by reliability principles and food safety criticalities. The effectiveness of maintenance is heavily dependent on the ability to identify the different CCPs existing in a production line, and to define the critical control parameters, processes and parts that characterize each CCP. The ability to identify the Biological, Chemical and Physical risks associated with the different critical control elements, to weight them and to design specific maintenance activities, represents a real competitive weapon in the hands of food companies.
INTRODUCTION
The industries involved in processing and packaging liquid foods, such as milk, fruit juice etc., have always been conscious of the need to establish and maintain the highest standard of hygiene. The competition in the food industry leaves very little room for error for a company when estimating production costs and the influence of product safety and production effectiveness. As a result of these trends the organization of maintenance has an important role to play in developing competitive advantage.
When we consider the process of transferring, pasteurising and sterilizing a liquid food such as milk, we know and have learned from experience, that bacterial contamination of milk is often caused by the equipment used. The ‘state of the art’ of the systems used to sterilise the container or packaging materials (PM), of the sensors used to monitor critical parameters such as temperature, flow rate and concentration of fluids, and of automation, represents an important factor in determining container sterilisation effectiveness.
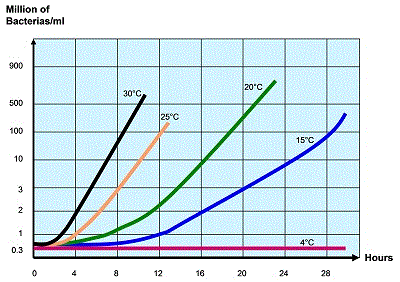
Figure 1 shows bacterial growth, following a contamination, in raw milk, the graph indicating the rate of bacterial development at different temperatures.
Aseptic and Extended Shelf Life (ESL) Filling Equipment Criticalities
Equipment and technologies used to pack aseptic liquid foods are rather complex. Since packaging of fresh foods has a lower complexity, this paper will mainly show the technical criticalities of aseptic packaging systems. Aseptic packaging can be defined as the filling of a commercially sterile food product into a sterile container under aseptic conditions, followed by hermetical sealing so that re-infection is prevented.
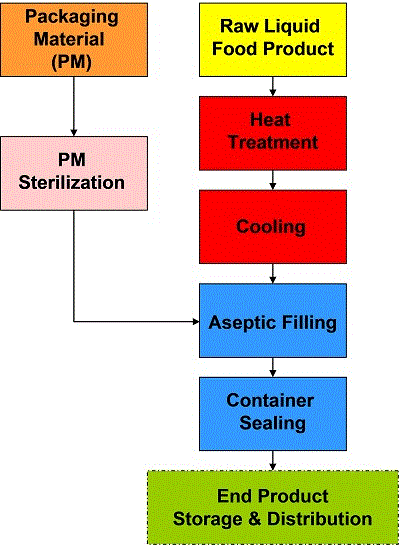
As shown in Figure 2, aseptic processing and packaging comprises the following phases:
- Sterilisation (direct or indirect heating) of products before filling;
- Sterilisation of packaging materials or containers;
- Aseptic filling:
- – sterilising the aseptic filler before operation,
- - maintaining sterility during production
- Package filling, forming, sealing and cutting;
- Production of filled hermetic containers.
In aseptic packaging systems PM is sterilised by different methods. Hydrogen peroxide (H2O2), with concentrations of up to 35%, temperatures up to 80°C and PM contact times of up to 15 sec., has been found to be successful for in-line and continuous aseptic packaging. Due to legal restrictions the end food product must not contain H2O2 in quantity greater than 0.5 ppm. (parts per million). This is the reason why PM sterilisation systems must not only provide an effective sterilisation circuit, but also a drying circuit able to remove, mechanically and/or by heat, the H2O2 residues on surfaces in contact with food product. Different methods of PM sterilisation are currently used, but sterilisation efficiency should be established in terms of numbers of logarithmic cycle reductions of the most resistant micro-organisms.
PM is usually sterilised either:
- inside the filling equipment. or
- externally, and then introduced aseptically into the aseptic zone of the aseptic filler.
Micro-organism inactivation has traditionally been carried out by heating. H2O2 is one of the most widely used chemicals for sterilising PM. To sterilise PM surfaces (in contact with food), the first successful aseptic filling system used a combination of H2O2 and heat. Many aseptic packaging systems use H2O2 at concentrations varying from 30 to 35%, followed by hot air (at 60–125°C) to increase the sterilising effect and to dry H2O2 residues from packaging materials and other food contact surfaces. Sterilisation performance increases with both peroxide concentration and temperature.
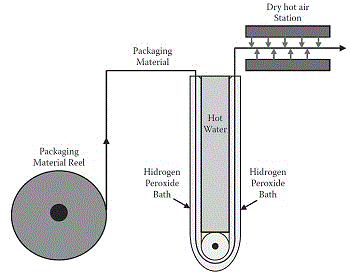
Figure 3 shows the PM reel being unwound and the PM dipped into an H2O2 bath where it is sterilized by immersion.. At the H2O2 bath out-feed a dry hot air station produces air knives, at a temperature of 100-130°C, able to dry H2O2 residues and improve PM sterilisation efficiency. In this type of system,
PM sterilisation efficiency depends on:
- Immersion time of PM into the H2O2 bath
In this system this is normally a fixed parameter dependent on:- length of hydrogen peroxide bath, - equipment speed, and
- volume of package
- H2O2 temperature and concentration;
- normally established by the equipment supplier, but they are usually in the range of 30-35% (concentration) and 60-80°C (temperature);
- Hot air (to dry H2O2 residues on PM) temperature and flow.
- established by the equipment supplier.
In this equipment unit, we have several critical components and parameters:
a) Packaging material guides
Different drive rollers and guides enable PM to be guided both outside and inside the H2O2 bath to avoid friction with metallic parts and contact with hot surfaces. Smooth and constant PM running or sliding within these units is important for its sterilisation and to avoid damage on internal and external surfaces (caused by scratches and pinches).
b) H2O2 temperature and concentration
The pre-set temperature of the H2O2 is indirectly achieved through heat generated and irradiated by an inner water bath heated up by a group of heating elements. H2O2 concentration can be automatically monitored by instruments, able to measure its density variation, or manually by the equipment operator.
c) Hot air station to dry and sterilize the PM
Air flow rate and temperature are two critical parameters of PM sterilisation efficiency. The gas phase, obtained by evaporation of a solution of H2O2 heated with hot air, has wide potential applications in sterilisation. Other criticalities may be introduced by burnt PM and polyethylene residues that can produce scratches on the side of the packaging material that comes in contact with food product.
Maintenance, calibration and cleaning of drive rollers and guides, and of thermo-regulators on mechanical components represent the tool to avoid the biological hazard produced by a poor PM sterilisation and by scratches and pinches on PM.
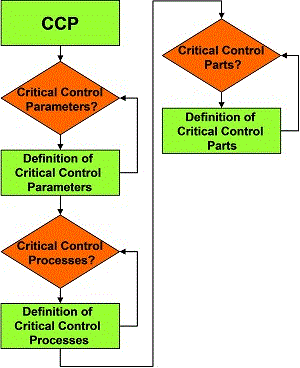
As indicated in Figure 4, through Hazard Analysis and Critical Control Points (HACCP) methodology all critical machine parts and components (CCPs) that have negative effects on food product safety have to be identified together with the risks associated with different failure modes. HACCP identifies and assesses specific hazards, estimates risks and establishes control measures that ensure product safety – through problem prevention and control rather than reliance on end-product testing and traditional inspection methods. Machine parts or components, in which faults may produce biological, chemical or physical hazard, are examined to devise critical control limits and preventive maintenance countermeasures.
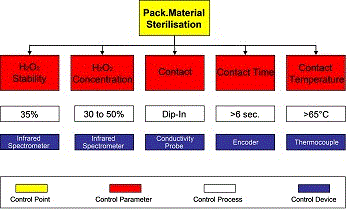
Figure 5 shows an example of an important critical control point that needs be taken into such consideration, i.e. PM sterilisation.
To carry out this activity effectively, it is important to identify:
- The critical control parameters
Normally the physical magnitudes, such as concentration, temperature, pressure and so on, that determine the execution of the equipment function that needs to be brought under under control; - The critical control processes
The processes used to control critical parameters; - The critical control devices
The critical components or parts used to control the processes and that need to be regularly checked or inspected.
The team carrying out this activity has to identify and define all potential parameters, processes and parts that are involved in establishing an effective control of the critical point under consideration. PM sterilisation is a chemical process which has five control parameters regarding:
- The concentration of the chemicals;
- The stability of the chemicals (of their concentration, pH, etc)
- The contact between the chemicals and the PM used;
- Chemical-PM contact time;
- The temperature of the chemicals used.
The quality of the chemicals used is normally controlled by the supplier, but periodical, parallel quality control must be carried out by the user to ensure the conformity of the product to declared standards and specifications. The user should, in particular, monitor the stability of H2O2 to avoid an unwanted variation of concentration, which is measured either by the equipment operator or by laboratory staff. In the latest plant this parameter is controlled by an automatic system such as an infra-red spectrometer, which provides a continuous and in-line measurement of H2O2. The H2O2 concentration can be measured over the entire pH range, over a wide concentration range, and with high precision and accuracy (errors less than 1%). The system consists of a bypass in which some electrodes are positioned and electronically controlled. The H2O2 consumption is measured by the equipment operators or by automatic systems. If the PM is dipped in the H2O2 bath, contact time depends on equipment design (length of bath and equipment speed). Since this process is a constant one this parameter does not need to be measured. An optical device, such as an absolute encoder, measures the movement of the PM relative to the machine driving system.
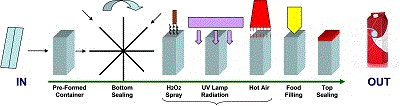
If PM is sterilised by a spraying system combined with a UV light, then a sensor should ensure contact time and radiation exposure. Contact temperature higher than 65 degrees is another critical parameter normally controlled by a thermocouple. The systems used to control process parameters must be examined to identify criticalities for which maintenance procedures need to be designed. Other PM sterilisation processes, which start from a pre-formed container instead of a PM reel, make use of the technology shown in Figure 6.
The pre-formed container enters the filler, and after the container is bottom-sealed a spray nozzle, injects a 2% concentration of H2O2 into the package. At the next station UV radiation reduces microbial contamination inside the package. The synergy established by a combination of H2O2, UV radiation and hot air (blown into the package at the next stage of the process) completes sterilising and drying of the package material for product filling. After final top-sealing the container is conveyed to the filler out-feed and to downstream plant.
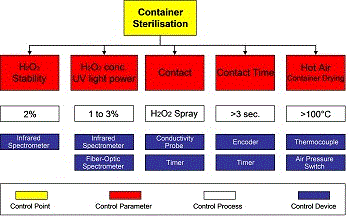
As indicated in Figure 7, with this technology we still have some other different criticalities that need to be brought under control via a reliable maintenance design programme:
- Hydrogen peroxide spray
The H2O2 concentration, the air pressure used to spray sterilisation solution into the package and the microfiltration of pressurized air, are some of the criticalities that need to be monitored via regular maintenance. - UV lamp radiation
The electrical parameters of the UV lamp power supply, and the feedback signal from the UV light radiated into the package need to be monitored to avoid low sterilization efficiency. - Hot air to dry and sterilise the package
Sterile air pressure/flow and temperature are some of the critical parameters that need to be monitored to avoid low sterilisation efficiency and anomalous H2O2 residues. - Filling station
There are different systems available to achieve a smooth and precise filling, but in any case, to avoid package integrity problems, it is mandatory to avoid product residues on the top sealing area of the package. Also in this case, maintenance design will play an important role in ensuring quality and reliability. - Package sealing
The technology used to achieve package bottom and top sealing makes use of heating bars, or ultrasonic or induction heating elements.
Other criticalities are dependent on: package position and stability, pressure of sealing jaws, heat generation and transfer to the package sealing area. Nowadays, many critical parameters have been brought under constant control by the installation of sensors and transducers that, connected to automatic control systems, facilitate a reliable monitoring activity. Nevertheless, despite the use of these modern technologies maintenance continues to play a fundamental role in keeping food criticalities under control. Corrections for mechanical wear, and adjustment and calibration of physical parameters can be undertaken only through a reliable maintenance design programme.
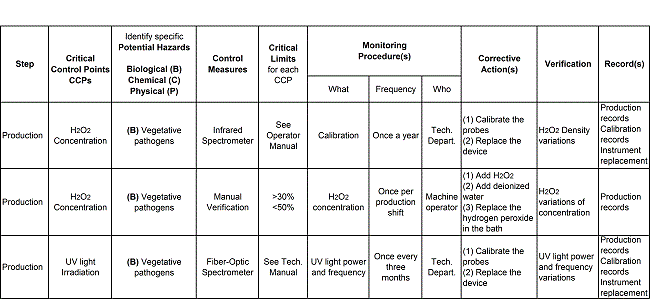
Table 1 shows the hazard analysis summary table for two critical control parameters:
- H2O2 concentration (automatic and manual), and
- UV irradiation.
The table summarizes the main activities aimed at bringing these two criticalities under control.
For each critical control parameter, different control processes must be found (i.e. the processes used to control the physical parameters). The question to answer at this stage is ‘What process enables control of this parameter?’ For each critical control process, different parts and component must be used to control each critical process (these are normally the parts, components, groups of components or instruments used to control the process). The question to answer at this stage is: ‘How can this process be controlled?’ Finally, as soon as we have identified the critical parameters, processes, and parts, we are in a position to design the maintenance activities to control the critical parts and components.
For critical operational practices (pre- or post-production, or during production) that are directly linked to Biological (B), Chemical (C), or Physical (P) hazards, potential deviations need to be identified, together with critical limits. H2O2 is normally the chemical used to sterilize packaging material, product pipes and the environment where package forming, filling, sealing, and cutting take place. Since H2O2 concentration is the most critical parameter to control, a lowest concentration measure, to avoid biological hazard as a consequence of low sterilization efficiency, must be carefully defined. On the other hand, a highest concentration threshold must also be defined, to avoid the risk of explosion resulting from chemical reactions due to H2O2 contamination contact with metal fragments or impurities.
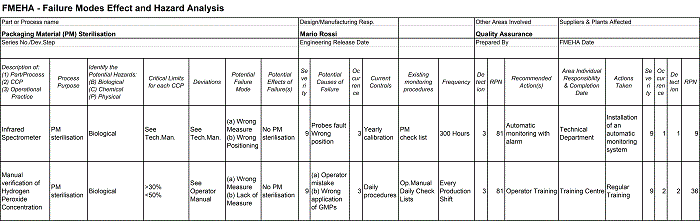
Table 2 shows the application of Failure Modes Effect and Hazard Analysis (FMEHA) to the H2O2 concentration that affects the CCP PM sterilisation. FMEHA integrates Failure Modes and Effect Analysis (FMEA) and HACCP principles, and provides a tool able to measure the criticalities associated with equipment reliability and food product safety. While the use of an automatic alarm enables a drastic reduction of the Risk Priority Number (RPN) from 81 to 9, the manual control of H2O2 concentration, carried out by the equipment operator, represents a risk and produces an RPN four times higher.
IN CONCLUSION
The identification of each equipment CCP must be followed by the characterization of the CCP through the proper definition of its critical control parameters, processes, and parts. The use of the Hazard Analysis Summary Table, together with FMEHA, enables identification and measurement of the criticalities, in order to compare the risk of each CCP, control parameter, process, and part. As result of this process we obtain a global view of all the critical elements, that are linked together and that determine a full control of the CCP under examination.
About the author
 Dr.Sauro Riccetti has carried out research on maintenance and process engineering in the food industry. His experience in Tetra Pak Italy, as Training Manager, Customer Service Director and
Business Development Director, and his involvement in improvement projects for the food industry have enabled him to gain a wide experience in maintenance and process engineering in that sector.
Dr.Sauro Riccetti has carried out research on maintenance and process engineering in the food industry. His experience in Tetra Pak Italy, as Training Manager, Customer Service Director and
Business Development Director, and his involvement in improvement projects for the food industry have enabled him to gain a wide experience in maintenance and process engineering in that sector.
He is also Adjunct Lecturer, teaching automatic machines for the food industry, at the University of Bologna.

